The rate of environmental pollution is increasing rapidly, resulting in water that is no longer safe to use if it does not go through any treatment system. This article will provide you with the most basic way to distinguish between Chlorine and Hydrogen Peroxide – the two most commonly used water treatment agents today.
The two most used water treatment agents today
The water treatment industry has been using Chlorine (Cl2) for over a hundred years. Chlorine gas was used in Belgium in 1903 to disinfect drinking water. In 1908, Jersey City, New Jersey, used the first liquid chlorine to disinfect water (sodium hypochlorite). In the 1950s, Eastern Europe was the first to use Hydrogen Peroxide (H2O2) to disinfect drinking water. H2O2 is known for its high oxidizing and bactericidal efficiency. Hydrogen Peroxide is not commonly used to disinfect drinking water, but its popularity seems to increase each year.

The use of chlorine in drinking water has always been popular in life until today. The key to chlorine is that it’s a great disinfectant. Chlorine can kill pathogens, microorganisms and bacteria, sometimes it can even harm humans if not used properly. Chlorine is an eye irritant to humans and an irritant to the human nasal passages and respiratory system. Chlorine is an excellent disinfectant but not a strong oxidizing agent, which is where H2O2 differs from chlorine.
Hydrogen Peroxide – Strong Oxidant
Hydrogen peroxide is a strong oxidizing agent. In fact, it is stronger than chlorine (Cl2), chlorine dioxide (ClO2) and potassium permanganate (KMnO4). In addition, through catalysis, Hydrogen Peroxide can be converted to Hydroxyl radicals (OH).
The main advantage that H2O2 has over Chlorine is that it does not form disinfection by-products (DBP’s) like Trihalomethanes (THM) which are known carcinogens. Whenever Chlorine is used, you must follow with the correct amount of granular activated carbon (GAC) to effectively remove DBP’s. On the other hand, Hydrogen Peroxide does not appear to produce such DBP’s.
Water treatment professionals may have to change their minds when comparing Hydrogen Peroxide to Chlorine because they are two different substances. One is a great disinfectant but the other is a great oxidizing agent and the way they are used varies greatly.
Chlorine requires contact time, typically 20 minutes for about 3.7 liters of water per minute of flow. On the other hand, Hydrogen Peroxide is not a good disinfectant but an excellent oxidizing agent. It does not require contact time because contact time only dilutes its oxidizing capacity.
Hydro Peroxide with Chlorine
Hydrogen Peroxide is not a good idea for disinfection just as Chlorine is not a good idea for Oxidation. If you need to disinfect surface water, especially if algae are present, then Chlorine is far superior to Hydrogen Peroxide. However, if you have very high levels of iron or sulfur then Chlorine is not a good choice, Hydrogen Peroxide is. Removal of iron and sulfur is best done by oxidation, and H2O2 is an excellent oxidizing agent.
Disinfection is the process of cleaning something with chemicals, to kill bacteria. While Oxidation and Reduction can be defined by adding or subtracting Oxygen to a compound; both reduction and oxidation polymerize, so they are called redox reactions.
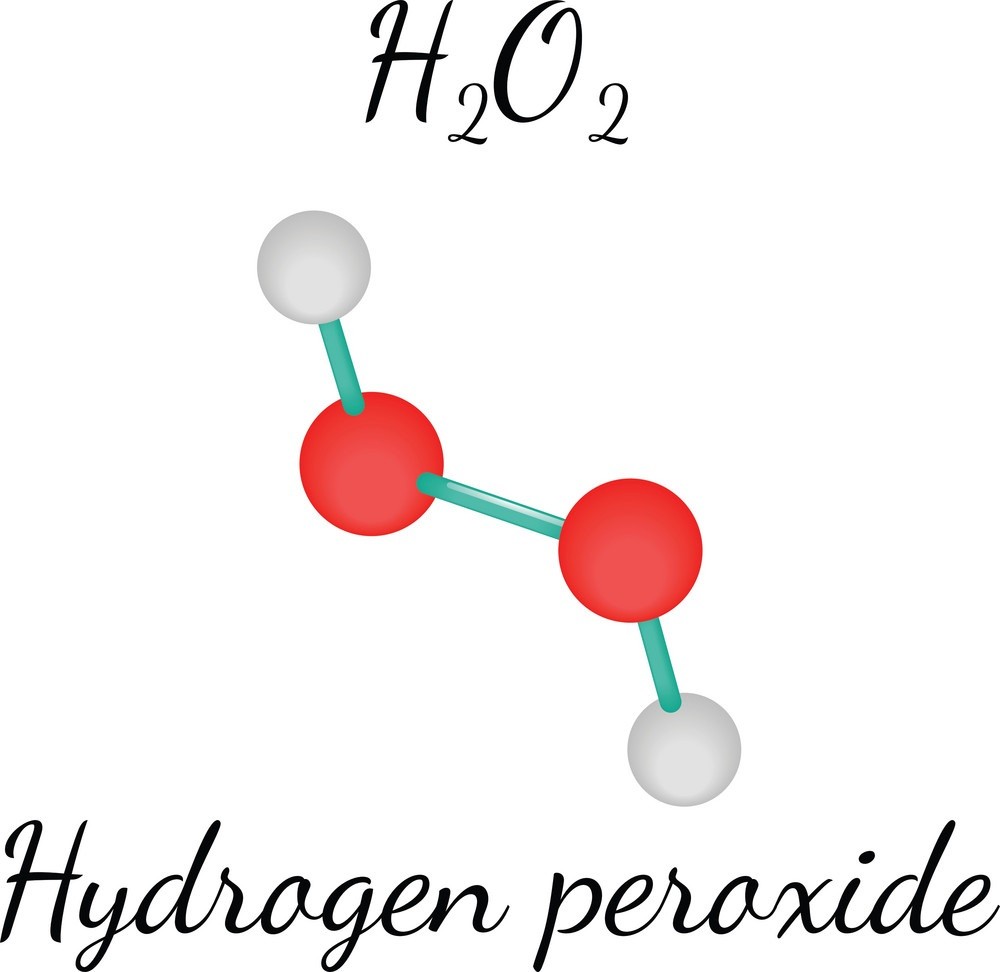
As stated, Hydrogen Peroxide has the chemical formula H2O2 and is an oxidizing agent similar to Oxygen but significantly stronger. The oxidizing activity of Hydrogen Peroxide is the result of the presence of an extra Oxygen atom relative to the structure of water.
Hopefully this knowledge can help you choose the right water treatment agent for the purpose of use. Don’t forget to follow Song Phung’s Website https://thietbinganhnuoc.com/ to update more knowledge about the water industry. In addition, you can order right at the Website to receive a promotional price.
Follow Fanpage: https://www.facebook.com/SongPhungthietbinganhnuoc/ to update new products.
More article: Troubleshooting water coolers
Translator: Duong Nguyen Hoang Khang


